थोरियम ईंधन चक्र
साँचा:asbox थोरियम ईंधन चक्र ( thorium fuel cycle) एक नाभिकीय ईंधन चक्र है जो थोरियम के समस्थानिक Th-232 का उपयोग उर्वर पदार्थ (फर्टाइल मैटेरियल) के रूप में करता है। रिएक्टर में Th-232 बदलकर यूरेनियम-२३३ बन जाता है जो नाभिकीय ईंधन है।
थोरियम के साथ नाभिकीय अभिक्रियाएँ
थोरियम भीगी हुई लकड़ी के समान है। जिस प्रकार भीगी लकड़ी को जलाने के लिए पहले भट्टी में सुखाना पड़ता है उसी तरह थोरियम को विखण्डनीय युरेनियम में बदलना पड़ता है ।"
— रतन कुमार सिन्हा, भारत के परमाणु ऊर्जा आयोग के भूतपूर्व अध्यक्ष[१]
In the thorium cycle, fuel is formed when साँचा:SimpleNuclide2 captures a neutron (whether in a fast reactor or thermal reactor) to become साँचा:SimpleNuclide2. This normally emits an electron and an anti-neutrino (साँचा:overline) by [[beta decay|βसाँचा:su decay]] to become साँचा:SimpleNuclide2. This then emits another electron and anti-neutrino by a second βसाँचा:su decay to become साँचा:SimpleNuclide2, the fuel:
- <math>\mathrm{n}+{}_{\ 90}^{232}\mathrm{Th}\rightarrow {}_{\ 90}^{233} \mathrm{Th} \xrightarrow{\beta^-} {}_{\ 91}^{233}\mathrm{Pa} \xrightarrow{\beta^-} {}_{\ 92}^{233}\mathrm{U}</math>
कचरा विखण्डन उत्पाद
Nuclear fission produces radioactive fission products which can have half-lives from days to greater than 200,000 years. According to some toxicity studies,[२] the thorium cycle can fully recycle actinide wastes and only emit fission product wastes, and after a few hundred years, the waste from a thorium reactor can be less toxic than the uranium ore that would have been used to produce low enriched uranium fuel for a light water reactor of the same power. Other studies assume some actinide losses and find that actinide wastes dominate thorium cycle waste radioactivity at some future periods.[३]
ऐक्टिनाइड कचरा
In a reactor, when a neutron hits a fissile atom (such as certain isotopes of uranium), it either splits the nucleus or is captured and transmutes the atom. In the case of साँचा:SimpleNuclide2, the transmutations tend to produce useful nuclear fuels rather than transuranic wastes. When साँचा:SimpleNuclide2 absorbs a neutron, it either fissions or becomes साँचा:SimpleNuclide2. The chance of fissioning on absorption of a thermal neutron is about 92%; the capture-to-fission ratio of साँचा:SimpleNuclide2, therefore, is about 1:12 — which is better than the corresponding capture vs. fission ratios of साँचा:SimpleNuclide2 (about 1:6), or साँचा:SimpleNuclide2 or साँचा:SimpleNuclide2 (both about 1:3).[४][५] The result is less transuranic waste than in a reactor using the uranium-plutonium fuel cycle. साँचा:Thorium Cycle Transmutation साँचा:SimpleNuclide2, like most actinides with an even number of neutrons, is not fissile, but neutron capture produces fissile साँचा:SimpleNuclide2. If the fissile isotope fails to fission on neutron capture, it produces साँचा:SimpleNuclide2, साँचा:SimpleNuclide2, साँचा:SimpleNuclide2, and eventually fissile साँचा:SimpleNuclide2 and heavier isotopes of plutonium. The साँचा:SimpleNuclide2 can be removed and stored as waste or retained and transmuted to plutonium, where more of it fissions, while the remainder becomes साँचा:SimpleNuclide2, then americium and curium, which in turn can be removed as waste or returned to reactors for further transmutation and fission.
However, the साँचा:SimpleNuclide2 (with a half-life of साँचा:val) formed via (n,2n) reactions with साँचा:SimpleNuclide2 (yielding साँचा:SimpleNuclide2 that decays to साँचा:SimpleNuclide2), while not a transuranic waste, is a major contributor to the long-term radiotoxicity of spent nuclear fuel.
यूरेनियम-232 प्रदूषण
Uranium-232 is also formed in this process, via (n,2n) reactions between fast neutrons and साँचा:SimpleNuclide2, साँचा:SimpleNuclide2, and साँचा:SimpleNuclide2:
- <math>\mathrm{n}+{}_{\ 90}^{232}\mathrm{Th}\rightarrow {}_{\ 90}^{233} \mathrm{Th} \xrightarrow{\beta^-} {}_{\ 91}^{233}\mathrm{Pa} \xrightarrow{\beta^-} {}_{\ 92}^{233}\mathrm{U}+\mathrm{n}\rightarrow {}_{\ 92}^{232} \mathrm{U}+2\mathrm{n}</math>
- <math>\mathrm{n}+{}_{\ 90}^{232}\mathrm{Th}\rightarrow {}_{\ 90}^{233} \mathrm{Th} \xrightarrow{\beta^-} {}_{\ 91}^{233}\mathrm{Pa}+\mathrm{n} \rightarrow {}_{\ 91}^{232}\mathrm{Pa}+2\mathrm{n} \xrightarrow{\beta^-} {}_{\ 92}^{232}\mathrm{U}</math>
- <math>\mathrm{n}+{}_{\ 90}^{232}\mathrm{Th}\rightarrow {}_{\ 90}^{231} \mathrm{Th} + 2\mathrm{n} \xrightarrow{\beta^-} {}_{\ 91}^{231}\mathrm{Pa}+\mathrm{n} \rightarrow {}_{\ 91}^{232}\mathrm{Pa} \xrightarrow{\beta^-}{}_{\ 92}^{232}\mathrm{U}</math>
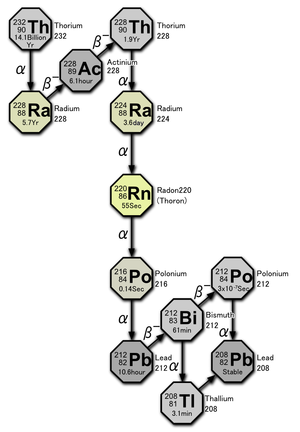
Uranium-232 has a relatively short half-life (साँचा:val), and some decay products emit high energy gamma radiation, such as साँचा:SimpleNuclide2, साँचा:SimpleNuclide2 and particularly साँचा:SimpleNuclide2. The full decay chain, along with half-lives and relevant gamma energies, is:
साँचा:SimpleNuclide2 decays to साँचा:SimpleNuclide2 where it joins the [[thorium series|decay chain of साँचा:SimpleNuclide2]]
- <math>{}_{\ 92}^{232}\mathrm{U} \xrightarrow{\ \alpha\ } {}_{\ 90}^{228}\mathrm{Th}\ \mathrm{(68.9\ years)}</math>
- <math>{}_{\ 90}^{228}\mathrm{Th} \xrightarrow{\ \alpha\ } {}_{\ 88}^{224}\mathrm{Ra}\ \mathrm{(1.9\ year)}</math>
- <math>{}_{\ 88}^{224}\mathrm{Ra} \xrightarrow{\ \alpha\ } {}_{\ 86}^{220}\mathrm{Rn}\ \mathrm{(3.6\ day,\ 0.24\ MeV)}</math>
- <math>{}_{\ 86}^{220}\mathrm{Rn} \xrightarrow{\ \alpha\ } {}_{\ 84}^{216}\mathrm{Po}\ \mathrm{(55\ s,\ 0.54\ MeV)}</math>
- <math>{}_{\ 84}^{216}\mathrm{Po} \xrightarrow{\ \alpha\ } {}_{\ 82}^{212}\mathrm{Pb}\ \mathrm{(0.15\ s)}</math>
- <math>{}_{\ 82}^{212}\mathrm{Pb} \xrightarrow{\beta^-\ } {}_{\ 83}^{212}\mathrm{Bi}\ \mathrm{(10.64\ h)}</math>
- <math>{}_{\ 83}^{212}\mathrm{Bi} \xrightarrow{\ \alpha\ } {}_{\ 81}^{208}\mathrm{Tl}\ \mathrm{(61\ m,\ 0.78\ MeV)}</math>
- <math>{}_{\ 81}^{208}\mathrm{Tl} \xrightarrow{\beta^-\ } {}_{\ 82}^{208}\mathrm{Pb}\ \mathrm{(3\ m,\ 2.6\ MeV)}</math>
Thorium-cycle fuels produce hard gamma emissions, which damage electronics, limiting their use in military bomb triggers. साँचा:SimpleNuclide2 cannot be chemically separated from साँचा:SimpleNuclide2 from used nuclear fuel; however, chemical separation of thorium from uranium removes the decay product साँचा:SimpleNuclide2 and the radiation from the rest of the decay chain, which gradually build up as साँचा:SimpleNuclide2 reaccumulates. The hard gamma emissions also create a radiological hazard which requires remote handling during reprocessing.
सन्दर्भ
- ↑ साँचा:cite news
- ↑ साँचा:cite web
- ↑ साँचा:cite web
- ↑ साँचा:cite web
- ↑ साँचा:cite web Thermal neutron cross sections in barns (isotope, capture:fission, f/f+c, f/c) 233U 45.26:531.3 92.15% 11.74; 235U 98.69:585.0 85.57% 5.928; 239Pu 270.7:747.9 73.42% 2.763; 241Pu 363.0:1012 73.60% 2.788.